April 2007 Meeting
Please join us at the three hundred and sixty-ninth meeting of the American Chemical Society Susquehanna Valley Section. The meeting will be held on Wednesday, 4 April 2007 in the Fitzgerald Room in the Sheehy-Farmer Campus Center on the King's College campus in Wilkes-Barre, PA. The speaker will be Dr. J. Martin Bollinger, Jr. from Department of Biochemistry and Molecular Biology and Department of Chemistry at The Pennsylvania State University. He will present "Oxygen activation by a mixed-valent diiron cluster and C-H cleavage by an iron-superoxide intermediate in the glycol cleavage reaction catalyzed by myo-inositol oxygenase".
Reception and Dinner: 6:00 PM in the Fitzgerald Room.
Dinner will be served with a selection of either orange peel chicken with jasmine rice or marinated London broil with wild brown rice (vegetarian option available). The cost will be $12.50 per person. Those interested in attending the dinner please contact Ron Supkowski with your choice at 570-208-5900 x5733 or ronaldsupkowski@kings.edu on or before Friday, 30 March 2007.
Meeting: 7:45 PM
Directions: see below
Oxygen activation by a mixed-valent diiron cluster and C-H cleavage by an iron-superoxide intermediate in the glycol cleavage reaction catalyzed by myo-inositol oxygenase
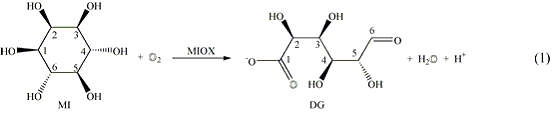
myo-Inositol oxygenase (MIOX) catalyzes the ring-opening, glycol-cleaving, four-electron oxidation of cyclohexan-1,2,3,5/4,6-hexa-ol (myo-inositol, MI) to D-glucuronate by a single equivalent of dioxygen (Eq. 1). The reaction initiates the only pathway in humans for catabolism of MI, the sugar backbone of cell-signaling phosphoinositides. Recent spectroscopic and kinetic studies on the mouse kidney enzyme produced in Escherichia coli have established that MIOX contains a dinuclear non-heme iron cluster.1,2 Unlike all non-heme diiron oxygenases and oxidases characterized prior to MIOX, which activate dioxygen with fully-reduced [diiron(II/II)] cofactors,3 MIOX activates O2 with the mixed-valent (II/III) form of its cofactor.2 The diiron(II/III) cluster directly coordinates the substrate, most likely leading to deprotonation of the C1-hydroxyl group, to activate the substrate.1 The MIOXII/IIIMI complex reacts with O2 to form a (formally) (superoxo)diiron(III/III) complex, G, which is characterized by a rhombic g = (2.05, 1.98. 1.90) EPR signal and accumulates only when the substrate contains deuterium.4 G converts to a second intermediate, a diiron(II/III)-containing complex, H, which has a g = (1.92, 1.76, 1.54) EPR signal and accumulates in the reaction with either protium- or deuterium-containing substrate.2,4 The G-to-H conversion is associated with a substrate deuterium kinetic isotope effect of 8-16, indicating that G abstracts hydrogen from the substrate.4 The kinetic and spectroscopic data on MIOX provide the first example of O2 activation by a mixed-valent diiron cluster and the most direct evidence yet reported for C-H cleavage by a superoxide-level metalloenzyme intermediate.
- Xing, G., Hoffart, L. M., Diao, Y., Prabhu, K. S., Arner, R. J., Reddy, C. C., Krebs, C. & Bollinger, J. M., Jr. (2006) Biochemistry doi 10.1021/bi0519607.
- Xing, G., Barr, E. W., Diao, Y., Hoffart, L. M., Prabhu, K. S., Arner, R. J., Reddy, C. C., Krebs, C. & Bollinger, J. M., Jr. (2006) Biochemistry doi 10.1021/bi0526276.
- Krebs, C., Price, J. C., Baldwin, J., Saleh, L., Green, M. T. & Bollinger, J. M., Jr. (2005) Inorg. Chem. 44, 742-757.
- Xing, G., Diao, Y., Hoffart, L. M., Barr, E. W., Prabhu, K. S., Arner, R. J., Reddy, C. C., Krebs, C. & Bollinger, J. M., Jr. (2006) Proc. Natl. Acad. Sci. U. S. A. 103, 6130-6135.
Dr. J. Martin Bollinger, Jr. earned his BA in Chemistry from The Pennsylvania State University and Ph.D. in Biochemistry from MIT. After a postdoctoral fellowship at Harvard Medical School, Dr. Bollinger joined the Penn State University faculty where he is currently Associate Professor in both the Department of Biochemistry and Molecular Biology and the Department of Chemistry.
Directions to Parking for the Sheehy-Farmer Campus Center at King's College
- From Route I-81, take Exit 170B (formerly Exit 47B) which will put you on Route 309 North.
- Follow Route 309 to exit 3 (Wilkes Barre/Plains).
- Turn left off the exit ramp onto North River Street. Proceed about 1.5 miles, and you will see the Luzerne County Courthouse on your right. Continue through the light at the courthouse.
- Turn left at the next street, West Jackson Street. There is no traffic light at West Jackson Street, so do not go as far as the next light.
- Go through the stop sign and at the first traffic light, turn right onto North Main Street.
- Turn right at the next light onto West Union Street.
- ~ About halfway down the block on your right you will see the entrance to a parking lot. Enter where the black and gold gate is raised. This part of the lot is paved ˙ã at the end of this row you can turn right and find plenty of additional parking on the gravel section of the lot.
- As you pull into this lot, you are looking at the back of the Sheehy-Farmer Campus Center, where your event is being held. It is a three-story building. You can walk to the front of the Campus Center by following the lane that runs along the left-hand side of the building (globe lights line the walkway).
- Your event is taking place on the 3rd floor of the Sheehy-Farmer Campus Center. The elevator is around the corner to your left after you enter the building, past the stairwell and large painting.
- For directions and a map of the campus, visit www.kings.edu/aboutkings/resources/directions .







.png)
.png)
.png)



